Product id: Good machine learning 2025 practices fda
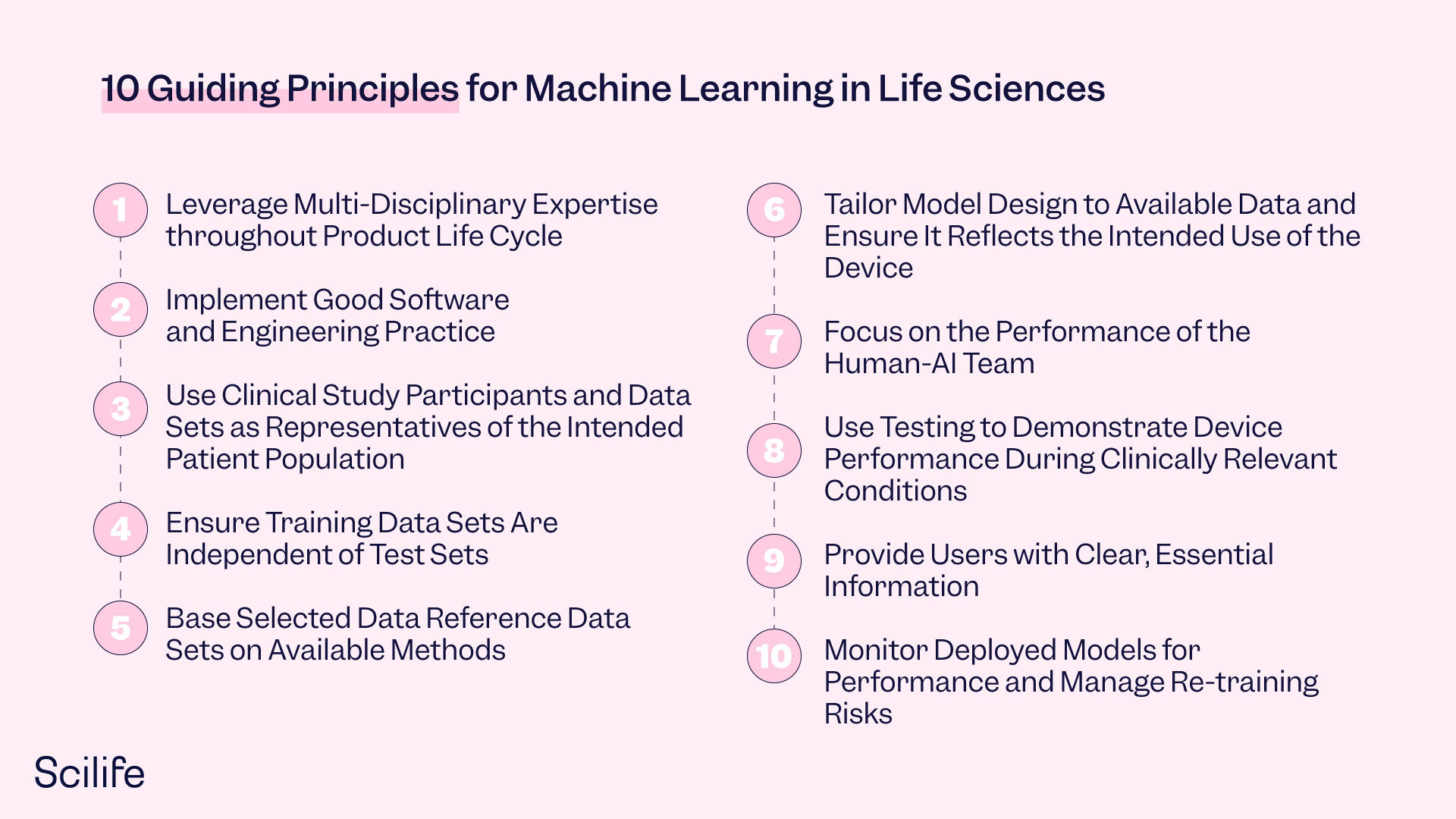
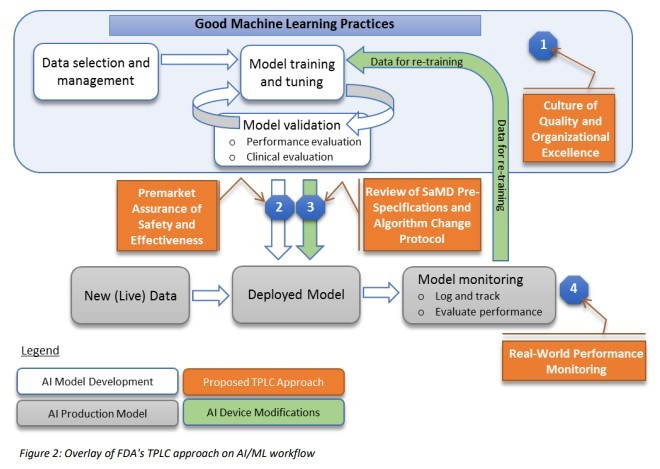
A dummy s guide to FDA good machine learning practices Hidden 2025, Guiding Principles GMLP 2025, Good Machine Learning Practice for Medical Device Development 2025, GOOD MACHINE LEARNING PRACTICE FOR THE MEDICAL DEVICE TOTAL 2025, FDA Good Machine Learning Practices PPT 2025, US FDA Artificial Intelligence and Machine Learning Discussion Paper 2025, FDA proposed workflow to regulate machine learning algorithms 2025, FDA Good Machine Learning Practice Guidelines and Practical QiML 2 0 2025, Good Machine Learning Practice for Medical Device Development 2025, GOOD MACHINE LEARNING PRACTICE FOR THE MEDICAL DEVICE TOTAL 2025, What the Principles for Good Machine Learning Practice mean gliff.ai 2025, GMLP Good Machine Learning Practices for medical device 2025, Good Machine Learning Practices for Medical Devices Orthogonal.io 2025, Brendan O Leary posted on LinkedIn 2025, US FDA Health Canada and UK MHRA have Jointly Identified 10 2025, FDA Medical Devices on X 2025, Uday Veera on LinkedIn Good Machine Learning Practice for Medical 2025, Good Machine Learning Practice for Medical Device Development 2025, Predetermined Change Control Plans for Machine Learning Enabled 2025, Student Perspective The role of the FDA in the future of medtech 2025, FDA Issues Guiding Principles for Good Machine Learning Practice 2025, FDA Good Machine Learning Practices PPT 2025, The state of artificial intelligence based FDA approved medical 2025, Overlay of FDA s TPLC approach based on GMLP and artificial 2025, AI medical software how will the FDA deal with it 2025, Quality Systems and Good Machine Learning Practices PDF 2025, Untitled 2025, good machine learning practices BioWorld 2025, Article Headline 2021 11 23 ASSEMBLY 2025, FDA Good Machine Learning Practice Guidelines and Practical QiML 2 0 2025, FDA and global peers create guiding principles for AI ML medical 2025, FDA Good Machine Learning Practices PPT 2025, Artificial Intelligence and Machine Learning in Software as a 2025, Navigating Machine Learning Guidelines for Medical Devices Scilife 2025, FDA AI ML Medical Device Guidelines Potential QML Submissions 2025.
A dummy s guide to FDA good machine learning practices Hidden 2025, Guiding Principles GMLP 2025, Good Machine Learning Practice for Medical Device Development 2025, GOOD MACHINE LEARNING PRACTICE FOR THE MEDICAL DEVICE TOTAL 2025, FDA Good Machine Learning Practices PPT 2025, US FDA Artificial Intelligence and Machine Learning Discussion Paper 2025, FDA proposed workflow to regulate machine learning algorithms 2025, FDA Good Machine Learning Practice Guidelines and Practical QiML 2 0 2025, Good Machine Learning Practice for Medical Device Development 2025, GOOD MACHINE LEARNING PRACTICE FOR THE MEDICAL DEVICE TOTAL 2025, What the Principles for Good Machine Learning Practice mean gliff.ai 2025, GMLP Good Machine Learning Practices for medical device 2025, Good Machine Learning Practices for Medical Devices Orthogonal.io 2025, Brendan O Leary posted on LinkedIn 2025, US FDA Health Canada and UK MHRA have Jointly Identified 10 2025, FDA Medical Devices on X 2025, Uday Veera on LinkedIn Good Machine Learning Practice for Medical 2025, Good Machine Learning Practice for Medical Device Development 2025, Predetermined Change Control Plans for Machine Learning Enabled 2025, Student Perspective The role of the FDA in the future of medtech 2025, FDA Issues Guiding Principles for Good Machine Learning Practice 2025, FDA Good Machine Learning Practices PPT 2025, The state of artificial intelligence based FDA approved medical 2025, Overlay of FDA s TPLC approach based on GMLP and artificial 2025, AI medical software how will the FDA deal with it 2025, Quality Systems and Good Machine Learning Practices PDF 2025, Untitled 2025, good machine learning practices BioWorld 2025, Article Headline 2021 11 23 ASSEMBLY 2025, FDA Good Machine Learning Practice Guidelines and Practical QiML 2 0 2025, FDA and global peers create guiding principles for AI ML medical 2025, FDA Good Machine Learning Practices PPT 2025, Artificial Intelligence and Machine Learning in Software as a 2025, Navigating Machine Learning Guidelines for Medical Devices Scilife 2025, FDA AI ML Medical Device Guidelines Potential QML Submissions 2025.